In recent years, the textile industry has come under growing pressure to address the environmental and health impacts of its chemical use. Legal regulations and demands from major international retailers have prompted significant changes in textile manufacturing processes. This article examines the efforts to reformulate and replace products in order to reduce toxicity and hazardous substances, with a focus on the influence of campaigns such as Greenpeace’s Detox initiative, the formation of industry groups like ZDHC, and the role of regulatory frameworks such as REACh. By exploring the strategies and challenges of this transition, the article highlights the progress made and the ongoing efforts to create a more sustainable and safer textile industry.
Restricted substances lists
Chemicals are restricted by legal restrictions and by the requirements of international retailers (brands) who are the big buyers in the global textile market.
The intense involvement of international brands was catalysed by massive NGO pressure, in particular the Greenpeace Detox campaign in the early 2010s[1]. Significant progress has been achieved by the industry in the last decade, a fact which is even acknowledged by Greenpeace[2] (Fig. 1). Meanwhile, Greenpeace moved their focus to fast fashion. As a result of Greenpeace´s detox campaign, associations such as ZDHC[3] were established. The primary goal is to achieve zero discharge of hazardous chemicals in the manufacturing process. Initially, large global retailers and brands participated, followed by chemical industry players. Today, the who is who of the textile supply chain is supporting ZDHC[4].
The brands requirements are represented in RSLs (restricted substances lists) and M-RSL (manufacturer restricted substances list) for the producers. The ZDHC criteria for M-RSL[5] are shown in Table 1. This list is used by the majority of the brands. Some brands have added further chemicals and chemical groups to this list, having more stringent individual M-RSLs, eg. Inditex, Miroglio, Valentino, as well as the Detox Committed suppliers in Italy CID (Italian Detox Consortium)[6]. The OEKO-TEX® STeP/DETOX TO ZERO Chemicals List[7], for example, is used by some German retailers.
| Chemical Group | Example Chemicals | Usage/Concern |
| Aromatic Amines | Benzidine, p-Chloroaniline (PCA), p-Cresidine, ß-Naphthylamine (BNA) | Released from certain Azo Dyes, known for being carcinogenic |
| Chlorobenzenes | Monochlorobenzene, Dichlorobenzenes, Trichlorobenzenes | Used in dye production, harmful to aquatic life |
| Chlorophenols | Pentachlorophenol (PCP), Tetrachlorophenol (TeCP), Tetrachlorophenol (TriCP) | Used as preservatives or in biocides |
| Brominated Flame Retardants | Polybrominated Biphenyls (PBB), Polybrominated Diphenyl Ethers (PBDE) | Prevent or slow the spread of fire |
| Glycols and Glycol Ethers | Ethylene Glycol, Diethylene Glycol, Triethylene Glycol | Used as solvents or antifreeze agents |
| Halogenated Solvents | Trichloroethylene, Perchloroethylene | Used in cleaning and degreasing processes |
| Organotin Compounds | Tributyltin (TBT), Triphenyltin (TPT) | Used as stabilizers in plastics and as biocides |
| Perfluorinated and Polyfluorinated Chemicals (PFCs) | Perfluorooctanoic Acid (PFOA), Perfluorooctane Sulfonate (PFOS) | Used for water, oil, and stain repellency |
| Phthalates | DEHP, DBP, BBP, DINP, DIDP, DNOP | Used as plasticizers in polymers |
| Short-Chain Chlorinated Paraffins (SCCPs) | C10-C13 chlorinated paraffins | Used in metalworking and as flame retardants |
| Volatile Organic Compounds (VOCs) | Benzene, Toluene, Xylene | Used in coatings, inks, and adhesives |
| Alkylphenols (AP) and Alkylphenol Ethoxylates (APEOs) | Nonylphenol (NP), Octylphenol (OP), Nonylphenol Ethoxylates (NPEOs) | Used as surfactants, endocrine disruptors, harmful to aquatic life |
Transparency was introduced by publishing wastewater details of textile manufacturers, linking them with their respective brands, “representing 5557 suppliers around the world” [8],[9].
Commercial organisations such as Bluesign and Hohenstein or non-profit organizations such as GOTS launched their own set certification standards. Worth mentioning is also the increasingly popular circular Cradle-to-Cradle (C2C) standard[10]. C2C contain additional criteria such as the exclusion of heavy metals.
A new industry for testing of all the concerned chemicals has evolved. Some are independent, global player such as SGS and Intertek, while others are subsidiaries of dyes & chemicals suppliers (such as Texanlab, India). Enormous effort is also taken within the chemical firms with sophisticated modern analytical labs to monitor contaminations during manufacturing.
Chemical law REACh
On the legal side, the most significant chemical law regulation is REACh in the European Union. The intention was to protect consumers and workers. Of course, we all want safe products and we want consumers and workers out of harms way. REACh may have played a role in protecting consumers, but the negative implications should not be overlooked: REACh pushed manufacturing to other regions of the world who did not have comparable restrictions. REACh absorbed valuable R&D resources for non-innovative bureaucratic activities. REACh helped multinational players to build boundaries to regional players. REACh pushed the launch of new products to emerging, non-regulated markets. And REACH was often a show-stopper for research into novel applications areas.
We are now entering a phase of a more level playground – with more countries implementing a REACh-like legislation like in the in the European Union. The following countries have established chemical laws similar to REACh: United Kingdom (post Brexit), South Korea, Turkey, China, Japan, United States, Canada, Australia. In Brazil, India and Pakistan regulations are in the pipeline. This means in most of the important markets chemical laws will be in place. With the implementation of REACh-like legislation in important textile manufacturing markets there is no way for chemical producers to bypass REACh-like legislations anymore. The countries not having a direct REACH-equivalent law, such as Bangladesh, align their practices with international standards to facilitate trade.
REACh contributed to reducing hazardous chemicals in the European market; however, since textile manufacturing shifted to non-regulated markets, the overall effectiveness remains questionable. Effectively the RSLs of the big brands were far more effective since these had immediate global impact whereas REACh initially affected only a declining market in Europe and imposed additional burdens to Europe.
Approach to clean/replace products
So, how are products cleaned or replaced to reduce toxicity and hazardous ingredients in order to meet legal and brand-specific requirements?
Significant know-how and deep understanding of chemical processes is required to understand the causes for hazardous chemicals.
The solution is a mix of:
- Avoid the use of hazardous chemicals
- Purify products or related intermediates to remove hazardous chemicals
- Good process control in manufacturing to avoid byproduct formation of hazardous chemicals
In the following paragraphs some case studies are outlined.
Case study: PFAS
If hazardous ingredients are used a raw material, the chemistry has to be changed to AVOID. For example, PFCs (poly fluorinated carbons) or PFAS (per- and polyfluoroalkyl substances), Nonylphenolethoxylates (NPE), Alkylphenolethoxylate (APEO) entire groups of chemicals are banned and alternatives are needed.
California has recently enacted significant legislation to ban PFAS, due to their persistence in the environment and potential health risks. California will prohibit the manufacture, sale, or distribution of new textiles and apparel containing PFAS finishing of textiles.
In the case of PFAS replacement products are of other chemistry, e.g. silicon chemistry. Unfortunately, replacement products are not reaching the same quality levels in repellent applications. High performing outdoor jackets can nowadays be bought in China, but no longer in western branded stores who comply to PFAS-free product standards.
A total organic fluorine (TOF) test is often used as a proxy for PFAS because it measures the total fluorine content from all organic fluorinated compounds present in a sample. This approach can indicate the presence of PFAS and other fluorinated organic substances. Unfortunately, this method also captures F from other applications such as F of heterocyclic dyes where F is built into a reactive group. CF3 groups in dyes are included in the strict PFAS definition although their profile is totally different to polyfluoroalkyl substances, and would be subject to forced phase out.
Case study: aromatic amines in dyes
In many other areas, the chemicals under scrutiny are unintentional by-products / contaminations from synthesis. Here chemicals are either purified or contaminations reduced by special manufacturing routes using stringent process control, or a combination of both.
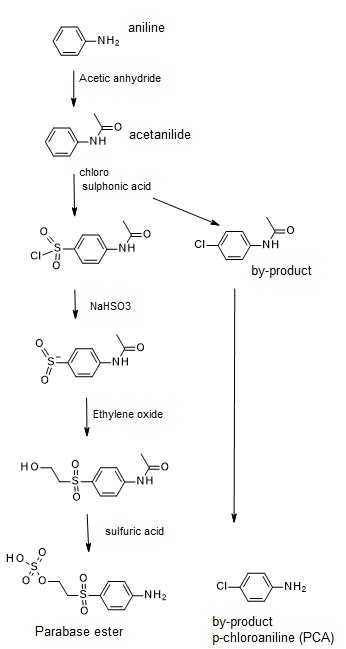
A notable example are restricted aromatic amines (Table 2). Such amines can occur in azo dyes, which are common in yellow, orange, red, navy blue and black dyes. Typically, the restricted arylamines are traces of byproducts from manufacturing, not intentionally used. Exceptions of intentional use are benzidine derivatives that were historically used in some direct dyes. However, with a few remaining exceptions, these dyes are no longer used in the market.
Of the restricted arylamines, p-chloroaniline (PCA) is a common contamination in reactive dyes. PCA can be formed as a by-product in the synthesis of a key intermediate (Parabase ester: Fig. 2) which is used in many reactive dyes including the biggest commodity products in the markets, such as Reactive Black 5 and Black mixtures. When the issue first came up 30 years ago, high amounts of PCA (hundreds of ppm) were found on the cloth, today levels can be controlled to 20-30 ppm levels. Good manufactures know how to fix the issue either by purification or by tight process control in intermediate manufacturing. Some players have launched alternatives products, entirely free of PCA, by using alternative intermediates. Such dyes are then promoted as PCA-free against a common detection limit. Unfortunately, many PCA-free dyes are significantly more costly, therefore having limited market success (the market is not paying for PCA-free unless the buyer explicitly request it). Huntsman (now Archroma) started such promotion initiatives. Meanwhile several other player launched similar products, so it is expected that price levels would become more affordable in the future.
The list of restricted amines is shown in Table 2. Acceptable limits are typically 20 ppm (= mg per kg of textiles) according to RSLs from ZDHC, Afirm and various others.
Aromatic amines are not present in the free form, they are built into the dye molecules. It is assumed that the fixed dye would undergo reductive cleavage by metabolization in human organism to release the amines[11]. Certain aromatic amines have been reported to be powerful carcinogens and mutagens[12]. The gradation of potency of the carcinogenic amines depends primarily on their hydrophobicity, and secondly on electronic and steric properties. The differentiation is known from science, yet all restricted amines in the list are treated in the same way, and the same limit apply for all. The setting of then limits in general is more political than scientific.
| Name of aromatic amine | CAS Number |
|---|---|
| 4-Aminobiphenyl | CAS No. 92-67-1 |
| Benzidine | CAS No. 92-87-5 |
| 4-Chloro-o-toluidine | CAS No. 95-69-2 |
| 2-Naphthylamine | CAS No. 91-59-8 |
| o-Aminoazotoluene | CAS No. 97-56-3 |
| 2-Amino-4-nitrotoluene | CAS No. 99-55-8 |
| p-Chloroaniline | CAS No. 106-47-8 |
| 2,4-Diaminoanisole | CAS No. 615-05-4 |
| 4,4′-Diaminodiphenylmethane | CAS No. 101-77-9 |
| 3,3′-Dichlorobenzidine | CAS No. 91-94-1 |
| 3,3′-Dimethoxybenzidine | CAS No. 119-90-4 |
| 3,3′-Dimethylbenzidine | CAS No. 119-93-7 |
| 4,4′-Methylene-bis-(2-chloroaniline) | CAS No. 101-14-4 |
| 4,4′-Oxydianiline | CAS No. 101-80-4 |
| 4,4′-Thiodianiline | CAS No. 139-65-1 |
| o-Toluidine | CAS No. 95-53-4 |
| 2,4-Toluylenediamine | CAS No. 95-80-7 |
| 2,4,5-Trimethylaniline | CAS No. 137-17-7 |
| o-Anisidine | CAS No. 90-04-0 |
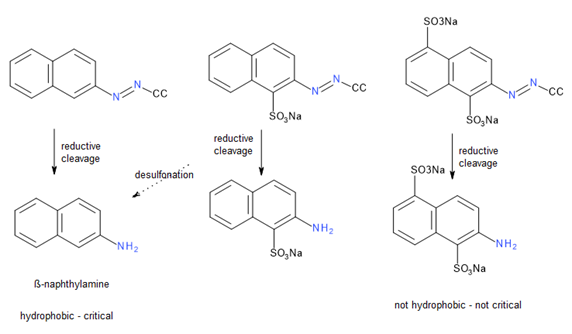
Aromatic amines with hydrophobic structures are potentially problematic when used as diazo components in azo dyes. Under test conditions, these amines can be cleaved to release free aromatic amines. Scientists understand how to design dye molecules to mitigate this issue: aromatic amines are not problematic when they are condensed into a heterocyclic ring or when they carry sulfo groups, as these modifications make them no longer hydrophobic.
Moreover, the test method is challenging in terms of reproducibility and requires proper analytical techniques and highly skilled operators. Frequently, we encounter false positives, and in some cases, this issue is inherent because certain amines are converted during testing, leading to the loss of a sulfo group and creating a critical amine in situ (Fig. 3). The situation is too complex to clearly communicate to retailers and brands, resulting in various suppliers withdrawing products due to false positive results, even though these products are not actually problematic.
As a bottom line, the issue of aromatic amines can be controlled by product selection, streamlining and good manufacturing practices.
Other potentially hazardous ingredients in dyes
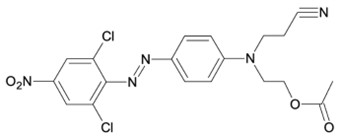
In some dye classes other hazardous chemicals can be present, such as chlorinated phenols (TriCP, TeCP, PCP) in certain disperse dyes or in triphendioxazine blue dyes. Chlorinated phenols are potentially critical, their presence may even indicate that dioxines could also be present. A known example for potential PCP contamination is Disperse Orange 30 (Fig. 4). The solutions is: take utmost care in intermediate manufacturing to avoid the formation of chlorinated phenols.
Heavy metals can be contained in some reactive dyes, Cu complexes are very common in trichromatic blues. The level of free Cu can be controlled by manufacturing to reach ETAD levels. Entirely Cu free azo dyes, as requested by Cradle to Cradle, would have lower light fastnesses. Other chromophore systems such as anthraquinone or triphendioxazine blues are challenging in their application performance and rarely used for trichromatic shades.
Indigo (vat blue 1) is the popular blue dye used for blue jeans and one of the biggest single dyes in the entire market. High amounts of aniline can be contained in indigo. Aniline is an essential raw material in the chemical synthesis of indigo. The contamination can be in the thousands of ppm. It is difficult to remove since it is captured inside indigo crystals. “Avoid” is not possible at reasonable cost levels (natural or enzyme made indigo is for alternative consideration). The solutions here is purification of aniline contamination by vacuum distillation in the manufacturing of pre-reduced indigo vat to significantly reduce aniline contents[13]. One supplier claims to reach aniline-free indigo in a patented process[14], whereas aniline-free means “below limits of detection according to industry standard test methods”[15] which is above the state of the art detection limit.
Conclusions
In conclusion, the textile industry has made significant progress in reducing toxicity and hazardous ingredients in response to regulatory pressures and consumer demands. Through the combined efforts of initiatives like Greenpeace’s Detox campaign, industry associations such as ZDHC, and legal frameworks like REACh, the sector has adopted comprehensive strategies to mitigate the use of harmful substances. Despite ongoing challenges, particularly with complex chemicals like PFAS and aromatic amines, the industry continues to innovate and implement rigorous process controls. These efforts not only aim to ensure consumer safety but also promote a more sustainable and environmentally responsible manufacturing process, reflecting the industry’s commitment to a safer future.
[1] https://www.greenpeace.org/international/act/detox/
[2] Greenpeace publication: “Self regulation: a fashion fairytale” (2021), https://www.greenpeace.de/publikationen/20211122-greenpeace-detox-fashion-fairytale-engl-pt1_0.pdf
[3] https://www.roadmaptozero.com/
[4] https://www.roadmaptozero.com/signatories
[5] https://mrsl-30.roadmaptozero.com/
[6] https://consorziodetox.it/wp-content/uploads/2022/02/RSL-_Natural-recycled-fibres-textile-WEB-EN.pdf
[7] https://www.oeko-tex.com/fileadmin/user_upload/DETOX_TO_ZERO_by_OEKO-TEX_R__-_Guideline_01.2022_EN.pdf
[8] https://www.detox.live/
[9] http://wwwen.ipe.org.cn/MapBrand/Brand.aspx?q=6
[10] https://c2ccertified.org/
[11] Stingley, R.L., Zou, W., Heinze, T.M., Chen, H., Cerniglia, C.E. (2010). “Metabolism of Azo Dyes by Human Skin Microbiota.” Journal of Medical Microbiology, 59(1): 108-114
[12] Benigni, R., Passerini, L. (2002). “Carcinogenicity of Aromatic Amines: From Structure-Activity Relationships to Mechanisms of Action and Risk Assessment.” Mutation Research/Reviews in Mutation Research, 511(3): 191-206.
[13] BASF patent EP523532 (1991), BASF patent US6428581(1998), Dystar patent WO2004/024826 (2002)
[14] Archroma patent WO2019030396 (2018)
[15] https://www.archroma.com/innovations/denisolpure-indigo-30liq