 Nobody knows what the future will bring and how fast. The future of coloured wool may be coloured sheep after all – Chinese scientists in Urumqi have are using already gene editing techniques to alter the coat colours of sheep[1]. However, the wool industry is a very conservative industry and the changes may not be so disruptive anytime soon.
Nobody knows what the future will bring and how fast. The future of coloured wool may be coloured sheep after all – Chinese scientists in Urumqi have are using already gene editing techniques to alter the coat colours of sheep[1]. However, the wool industry is a very conservative industry and the changes may not be so disruptive anytime soon.
Real and perceived environmental risks, pressure from retailers and legislation, are today the driver for change. The main challenges are metals in dyeing, especially related to chromium (VI), absorbable organic halogen (AOX) in shrink-resist finishing and pesticides in mothproof finishing. These environmental challenges and technical alternatives in wool dyeing & finishing are discussed in this article.
Wool Composition and Dyeing
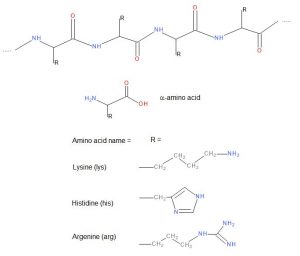
Wool is an animal fibre made of polypeptides, a biodegradable natural protein fibre. Wool is mainly from sheep hair. Other wool fibres are for example cashmere, angora and alpaca. Further examples of protein fibres are human hair and silk. Proteins are built of amino acids connected to each other by peptide bonds.
The dyeing and finishing of wool is based on several physical and chemical effects such as electrostatic interactions, van der Waals interactions and entropy driven hydrophobic interactions[2].
Amino groups in wool play an essential role in these interactions. The amino groups can form metal complexes with metals in dyes, form electrostatic interactions between protonated ammonium groups and sulfo groups in anionic dyes, and react with reactive dyes to form covalent bonds.
The amino acids in wool which carry amino functions are lysine (3.1 mol%), arginine (6.8 mol%), histidine (0.9 mol%) and terminal primary amino groups2. In addition, wool has many carbonamido groups in peptide bonds which can form hydrogen bonds with dyes.
To read the full article, please login. The full content of this article and all premium articles is available exclusively for site members.
Site membership is free. If you are an existing user, please login. New users may register below.


