Technical background of water and soil repellent finishing on textiles, and alternatives for the future
In a previous article, “Greenpeace puts pressure on outdoor brands to phase out hazardous chemistry”, we have discussed reports of Greenpeace and the German Umweltbundesamt (UBA) on hazardous fluorocarbons found in outdoor articles, and the progress of the ZDHC brands towards elimination of such hazardous chemicals.
In this article we discuss the technical and scientific background of water and soil repellent finishing on textiles, and will look into options for future, more sustainable technologies and chemistries.
State of the art in water and soil repellency, and what the consumer really needs
For water & soil repellent finishing on textiles various chemical solutions can be applied, of which perfluorocarbon (PFC) chemistry has become state of the art in performance. But this chemical substance class has been put under critical observation in regard to their toxicity and persistence in the environment. Certain types of PFCs, long chain PFCs such as PFOA (perfluorooctanoic acid), have been put on the black list by ZDHC brands and Greenpeace, while there are still different opinions to what extent and how fast this should be implemented.
 For a judgment for the technology, one has to differentiate between water and soil/oil repellency.For water repellency good alternatives to long-chain fluorocarbons are available, as discussed further in the context.
For a judgment for the technology, one has to differentiate between water and soil/oil repellency.For water repellency good alternatives to long-chain fluorocarbons are available, as discussed further in the context.
On the other hand, for oil, blood and soil repellency, no commercial product is known yet to match the performance of long-chain fluorocarbons. This fact is even acknowledged by Greenpeace in their study[1].
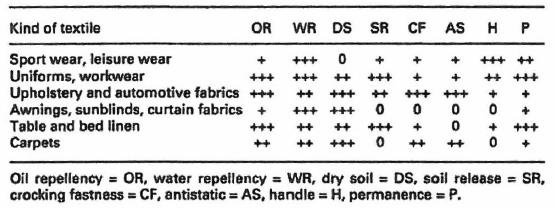
The following table[2] shows the technical requirements, differentiated by textile article class.
To read the full article, please login. The full content of this article and all premium articles is available exclusively for site members.
Site membership is free. If you are an existing user, please login. New users may register below.