Sustainable colouration concepts Part III. Issues in textile processing of cotton
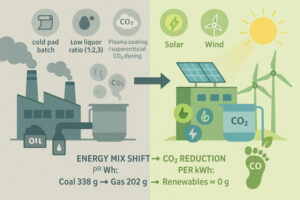
 In the previous article we have discussed an innovative water-free dyeing process for polyester, dyeing from supercritical CO2 (scf-CO2). Today we will look at cotton, which is a major contributor for polluted effluents. Cotton has ethical and ecological concerns, as well as requiring high water & energy consumption in the textile industry.
In the previous article we have discussed an innovative water-free dyeing process for polyester, dyeing from supercritical CO2 (scf-CO2). Today we will look at cotton, which is a major contributor for polluted effluents. Cotton has ethical and ecological concerns, as well as requiring high water & energy consumption in the textile industry.
Cotton is by far the most important natural, renewable fibre, used for textiles. Cotton and cotton blends are almost 40% of the total textile fibre consumption. The second biggest natural fibre is wool, from animal hairs, with only 2% share.
Cotton is not based on depletable raw materials such as crude oil or coal tar, compared to synthetic fibres like polyester or nylon. In the view of a peak oil scenario, this may be an important factor in the future.
To read the full article, please login. The full content of this article and all premium articles is available exclusively for site members.
Site membership is free. If you are an existing user, please login. New users may register below.